are your patientsTrapped By TGCT?
UNTANGLE THE TRUTH OF TENOSYNOVIAL GIANT CELL TUMOR (TGCT) AND THE STRUGGLES YOUR PATIENTS MAY BE FACING
Surgery every year is exhausting. We want more options to surgery. We want more proactive medicine rather than just waiting for significant regrowth.
— Person living with TGCT


TGCT, previously called pigmented villonodular synovitis (PVNS) or giant cell tumor of the tendon sheath (GCT-TS) is a locally aggressive neoplasm which can cause permanent joint damage, significant pain, swelling, decreased range of motion (ROM), and stiffness.1-3 While some cases of TGCT can be surgically resected and cured, others can be difficult to live with and challenging to treat.4
Truth is, TGCT can be harrowing1,5
Pain, joint stiffness, and physical limitations can be debilitating and life‑altering.5 Surgical resection is considered the standard of care. However, non‑surgical treatment options do exist and many patients could benefit from learning about them sooner in their journey.6-8
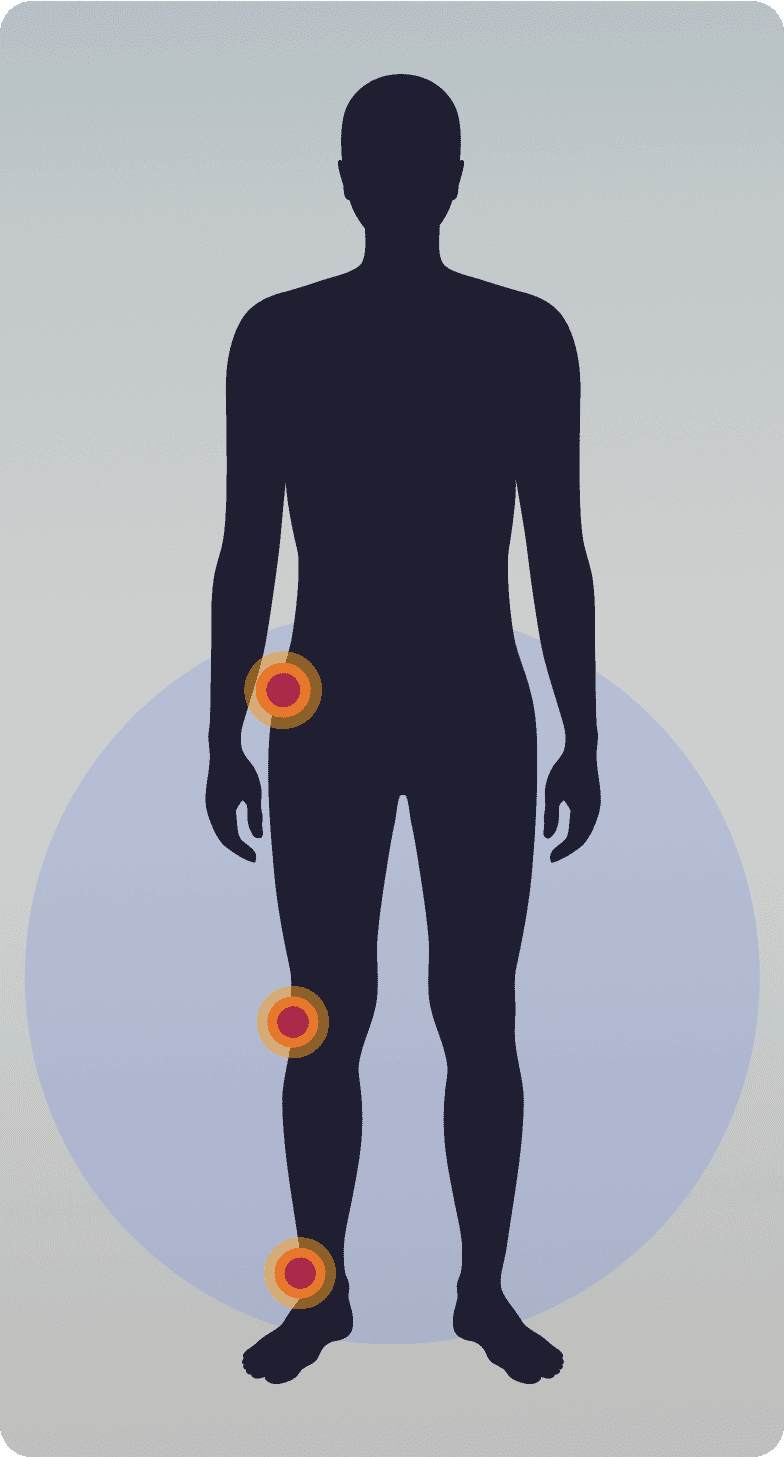
There are two types of TGCT1,2
Localized/nodular TGCT
More common9
Normally a single, well-defined tumor, usually nodular1,10
Typically smaller, ~2 cm in size1,11
Often does not cause pain or joint dysfunction1,2
Tumor location:Tends to impact smaller joints like hands, fingers, wrist, foot1,12
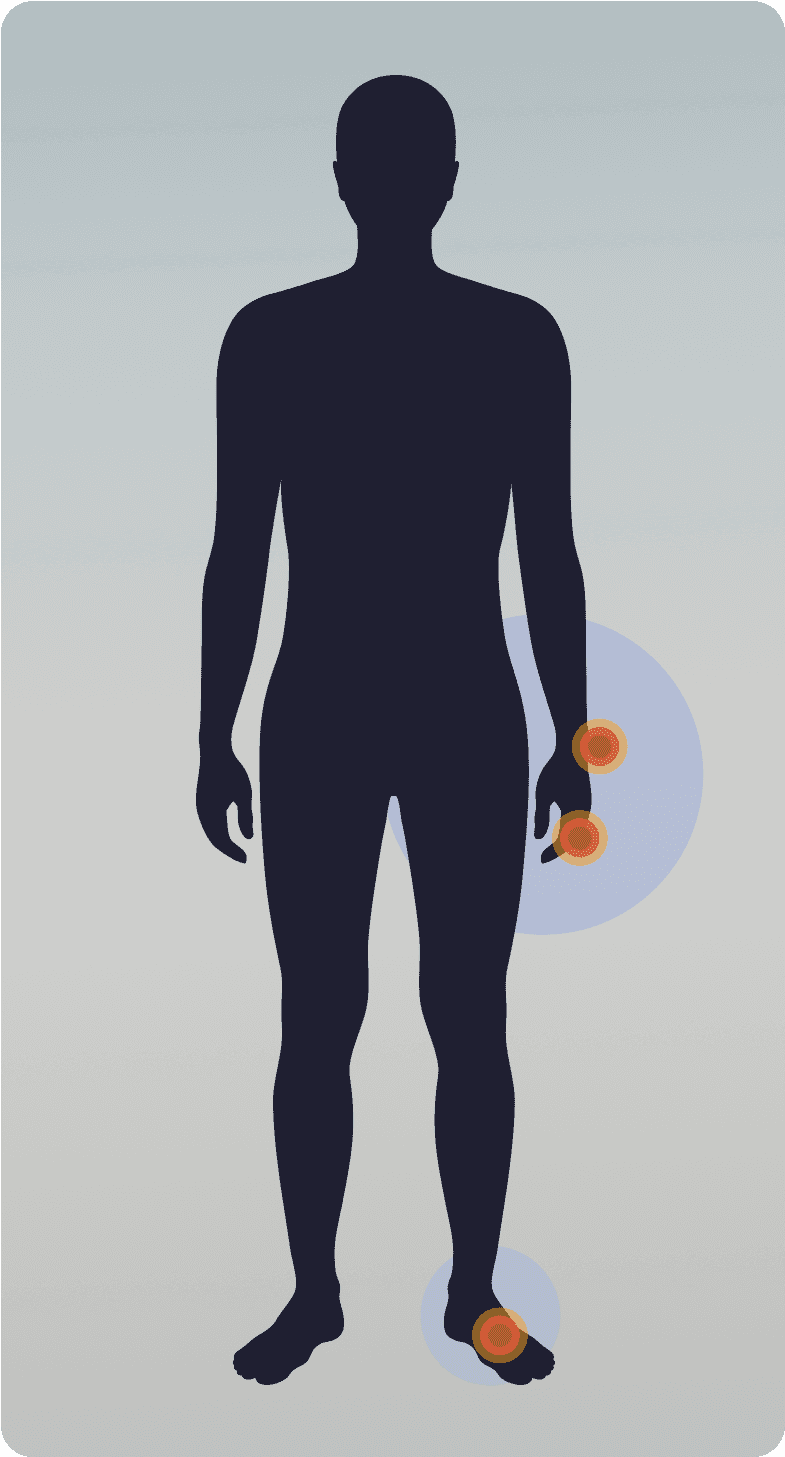
Diffuse TGCT
Less common9
Ill-defined tumors that develop outside of the joint13,14
Large tumors, >5 cm in size13
Can extend out of the joint and damage surrounding bone1,14
Tumor location:Tends to impact larger joints like knee, hip, ankle2,4
In a qualitative interview study of 20 participants with histologically confirmed TGCT in the knee, hip, or ankle:
80
%
reported pain6
85
%
reported swelling6
Occurring in a young patient population, TGCT can have major impact on Quality of Life (QoL) and physical function15
TGCT can be a lifelong condition with long‑term impacts resulting from both the disease and treatments.1,15 TGCT affects a relatively young population with the average age of diagnosis being 33 years old.5
The physical and emotional burdens of TGCT can be significant and potentially lifelong1,5
40%or more of
TGCT patients
reported using opioids and NSAIDs in their treatment history3
The TGCT patient journey can be long, frustrating, and fraught with delays15
Once diagnosed, understanding all of the options and getting appropriate treatment can be difficult.1 Surgical resection, usually the first approach, does not treat the hypothesized underlying genetic driver of the disease.6,15
DIAGNOSIS
It can take up to 3-4 years to get a TGCT diagnosis15
PRIMARY TREATMENT
Typically surgery—but it is not a cure for many patients with TGCT and tumors often return3,15
TGCT RECURRENCE
A lifetime risk for both diffuse-type and localized/nodular-type TGCT16
TREATMENT AFTER RECURRENCES
- Repeated surgical resections. This may not be an option or effective for all TGCT patients and can be debilitating3,15
- Systemic treatment is often not discussed until a patient has had multiple recurrences and surgical resections15
Patients often get stuck in a surgery loop and may not be aware of other treatment options.3,8

Truth is, the majority of patients with diffuse TGCT will experience recurrence16
Due to the locally aggressive nature of the tumor, complete resection is difficult to achieve and often results in residual disease, recurrence, and multiple surgical resections.3,4,7 Diffuse TGCT is often resected incompletely, resulting in high recurrence rates and worse clinical outcomes.17,18
LIFETIME RECURRENCE RATES16
diffuse
TGCT
up to
55%
localized/
nodular
TGCT up to
15%
With each recurrence, TGCT becomes increasingly difficult to manage.3,4 Repetitive surgical resections can lead to increasingly significant morbidity, including joint destruction and secondary osteoarthritis.3,4 Because of its tendency to recur, diffuse TGCT can become a chronic, lifelong condition for some.1,15
Morbidity risk increases with each subsequent resection, adding to the DIFFICULTY of managing TGCT7,8
The prolonged course of the disease and the need for multiple resections has been reported to contribute to further joint destruction and, in some cases, lead to premature joint replacement or amputation.4
Over
40%of
patients
who had surgery for TGCT underwent 2 or more surgeries3
Joint replacement is not a cure for TGCT and carries the risk of recurrence and complications1,7,19

Truth is, current TGCT treatment options are limited7
The standard of care for symptomatic TGCT is surgical resection, when it can be accomplished without significant morbidity and depending on tumor location and the extent of the disease.1,20 Resection of diffuse TGCT is associated with a high risk of recurrence, postoperative complications, and joint damage.1,4
Repeated surgical resections can lead to increased morbidity, including acceleration of secondary osteoarthritis, permanent joint damage, and other lifelong consequences.3,4
~50 %
of patients
with DIFFUSE TGCTremain symptomatic, even after SURGERY1,5
Patients with difficult-to-manage, symptomatic disease, or moderate or severe functional impairment, may be candidates for targeted systemic treatment if the expected benefits of resection are outweighed by the morbidity risk.1
The potential benefits of any medication or systemic treatment need to be carefully weighed against the risks and expected impacts on QOL.1
Tyrosine kinase inhibitors (TKIs) targeting the colony stimulating factor 1 (CSF1) pathway have shown a wide range of efficacy, but may be limited by toxicity.20
In TGCT, both the disease and the current treatment options can impact QOL.4 With TGCT being a potentially lifelong and challenging disease to manage, symptomatic and functional improvement are essential parts of treatment decision making.1
What can be done to help improve outcomes if TGCT recurs?
Since TGCT can be challenging to control with surgery alone, a multimodal approach, including systemic treatments should be considered.17 Collaborative care involving a multidisciplinary team including an orthopedic surgeon, medical oncologist, and other sarcoma experts can help improve patient outcomes.1
A multidisciplinary team is critical early in the patient journey especially for patients with symptomatic, recurrent, or unresectable TGCT—or simply for patients who want to understand all of their options.1,15
Get the latest TGCT management guidelinesInternational Consensus Recommendations for the Management of TGCT
With ongoing research
there ARE REASONS to be hopeful7
Sign up for more information
I’m interested in finding out more about TGCT from Deciphera.